|
|
|
Copper and Aluminium. Background for natural resources GEOG2110
From web pages:
|
Metallic element |
Symbol |
Atomic Number |
Atomic Weight |
Melting Point |
Boiling Point |
Specific gravity at 20°C |
Valency |
|
Copper |
Cu |
29 |
63.546 |
1,083.4°C |
2,567°C |
8.96 |
+1 or +2. |
|
Aluminium |
Al |
13 |
26.9815 |
660.37°C |
2,467°C |
2.6989 |
+3 |
Copper
and some of its alloys have been used by humanity since the Bronze Age. One of the first metals known to humans, free copper was probably mined in the Tigris-Euphrates valley as long ago as the 5th cent. B.C. Cyprus, from which the metal's name originally comes, was the primary source of copper in the ancient world.Sources and Ores
Small amounts of copper are found uncombined, particularly near Lake Superior in Michigan. Copper ores are found in various parts of the world. In the United States (the chief producer of copper) ores are mined in Arizona, Utah, Montana, New Mexico, Nevada, and Michigan. Copper ores are also found in Canada, South America (in Chile and Peru), S central Africa, Russia (in the Ural Mts.), and to a limited extent in Europe and the British Isles.
The principal ore of copper is
chalcopyrite, a sulfide of copper and iron, also called copper pyrite. Other important ores are chalcocite, or copper glance, a shiny lead-gray copper sulfide; bornite, a lustrous reddish-brown sulfide of copper and iron; cuprite, a red cuprous oxide ore; and malachite, a bright green carbonate ore. Azurite is a blue crystalline basic carbonate of copper found with other copper ores. Chrysocolla is a bluish-green copper silicate ore. Another important source of copper is secondary (scrap) copper, which is produced from discarded copper and copper alloys.Importance and Uses
Copper is present in minute amounts in the animal body and is essential to normal metabolism. It is a component of hemocyanin, the blue, oxygen-carrying blood pigment of lobsters and other large crustaceans. It is needed in the synthesis of hemoglobin, the red, oxygen-carrying pigment found in the blood of humans, although it is not a component of hemoglobin.
The chief commercial use of copper is based on its electrical conductivity (second only to that of silver); about half the total annual output of copper is employed in the manufacture of electrical apparatus and wire. Copper is also used extensively as roofing, in making copper utensils, and for coins and metalwork. Copper tubing is used in plumbing, and, because of its high heat conductivity, in heat-exchanging devices such as refrigerator and air-conditioner coils. Powdered copper is sometimes used as a pigment in paints. An important use of copper is in alloys such as
brass, bronze, gunmetal, Monel metal, and German silver. Compounds of copper are widely used as insecticides and fungicides; as pigments in paints; as mordants (fixatives) in dyeing; and in electroplating.Copper
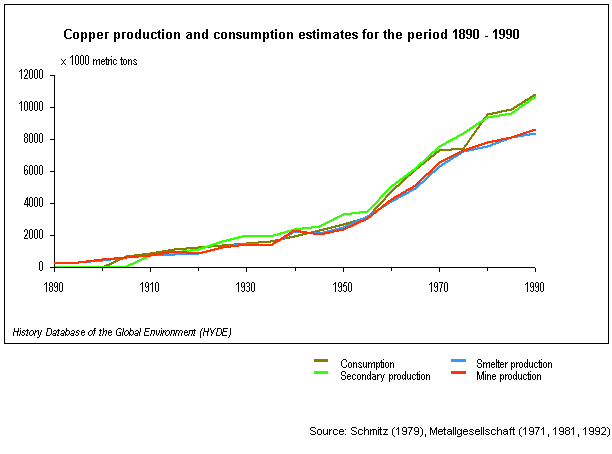
ranks third in world metal consumption after steel and aluminium. Major copper producing countries are Chile (24% of the global mine production) and the United States (19%). Copper use is dominated by electrical and electronic applications. Until the mid-50s copper consumption kept in pace with the production. Since then, the global copper consumption has steadily outgrown the production, in 1990 by 4%, and consumption patterns shifted towards the rapidly developing Asian countries. Copper production data are used for the calculation of SO2 emissions. Schmitz (1979) presents data for global non-ferrous metal production, including annual world primary copper mine and smelter production from 1700 to 1976. The sum of the regions is not equal to the world total given by the Metallgesellschaft, probably some countries were not included in the regional estimates. A good source for statistics on non-metal production after 1960 are the reports of the Metallgesellschaft. This table presents mine production of copper, primary and secondary copper production for the period 1890 - 1990 with a 5-year interval, and consumption for some regions from 1960 - 1990.Aluminium
is a silver-white metal with a face-centred cubic crystalline structure. It is a member of group IIIa of the periodic table. It is ductile, malleable, and an excellent conductor of heat and electricity. The pure metal is soft, but it becomes strong and hard when alloyed. Although less conductive than copper wire of the same diameter, Aluminium wire is often used for high-tension power transmission because it is lighter and cheaper. Although it is chemically very reactive, Aluminium resists corrosion by the formation of a self-protecting oxide coating. It is rapidly attacked by alkalis (such as lye) and by hydrochloric acid.Although it is the most abundant metal in the earth's crust (about 8% by weight), Aluminium does not occur uncombined but is an important constituent of many minerals, including clay,
bauxite, mica, feldspar, alum, cryolite, and the several forms of Aluminium oxide (alumina) such as emery, corundum, sapphire, and ruby. Commercially, Aluminium is prepared by the Hall-Héroult process, which consists essentially of the electrolysis of alumina prepared from bauxite and dissolved in fused cryolite. In an electric furnace an iron tank lined with carbon serves as the cathode and large blocks of carbon serve as the anode; the electric current generates enough heat to keep the cryolite melted. Molten Aluminium collects at the bottom of the tank, and oxygen is liberated at the anode. The anode is consumed as it combines with the oxygen to form carbon dioxide.Aluminium foil is used as a wrapping material. Aluminium powder is used in paints. A mixture of powdered Aluminium and iron oxide, called
thermite, is used in welding because of the large amount of heat liberated when it is ignited. The development of methods for colouring Aluminium led to its use in jewellery, on wall surfaces, and in coloured kitchenware. Important alloys of Aluminium include duralumin, Aluminium bronze, and Aluminium-magnesium; they are used extensively in aircraft and other industries.Although the metal was not isolated until the 19th cent., use of Aluminium compounds originated in antiquity. The Romans used various Aluminium compounds as astringents; they called these alum. Sir Humphrey
Davy and other chemists in the early 19th cent. recognised Aluminium as the metal and alumina as its oxide. H. C. Oersted succeeded in obtaining impure Aluminium in 1825, but Friedrich Wöhler had greater success and is usually credited with its first isolation, in 1827. H. E. Sainte-Claire Deville first prepared inexpensive pure metal in 1854 and set about perfecting a process for its commercial production. However, it was not until 1886 that the process by which Aluminium is produced today was discovered independently by C. M. Hall, a student at Oberlin College, and Paul Héroult, a French metallurgist. The process depends critically on the availability of cheap hydroelectric power.Aluminium
is the second most abundant metal element in the Earth's crust after silicon, but it has only been produced commercially for slightly more than 100 years. Nowadays, it is the second most widely used metal after iron and important in virtually all segments of the world economy (Plunkert, 1996). The United States was in 1990 still the world largest producer of primary aluminium, although its share in global production decreased from 40% in 1960 to 23% in 1990. Australia and Canada have emerged as major producers since then, and nowadays countries like Brazil, China, Norway, Venezuela and some Persian Gulf states are entering the world market. In secondary production a difference is made between new scrap (a.k.a. home or runaround scrap) and old scrap. Home scrap is recycled within the company generating the scrap and does not enter the market. Old scrap is generated by companies who do not want to or can deal with the scrap, and this part does enter the market (Plunkert, 1996). The recycling of e.g. softdrink cans, and car parts is still beginning to emerge in some industrialised countries. This table presents mine production of bauxite, primary and secondary aluminium production for the period 1890 - 1990 with a 5-year interval, and consumption for some regions from 1960 - 1990.